Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Our research interests focus on two groups of neuromuscular disorders (NMD): myopathies due to abnormalities of the myomatrix and of the nucleus. The long-term objective of our work is to propose relevant therapeutic options based on our knowledge of the genetic basis and of the underlying pathomechanisms at play in these rare diseases.
Research Project: Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Our team focuses on 2 groups of neuromuscular disorders: myopathies due to the defective myomatrix (collagen VI and other components of the extracellular matrix) and to defects of the myonucleus (Emery-Dreifuss muscular dystrophy and other striated muscle laminopathies due to mutations in the laminA/C gene or genes encoding components of nuclear membrane). These myopathies share some clinical features, notably prominent contractures, and constitute differential diagnosis[in1] .
These disorders are highly heterogeneous, clinically and genetically, and to date no treatment is available. Our previous work led us to identify the involvement of various genetic alterations and to develop tools (cellular and animal models) that are crucial for deciphering pathomechanisms, understanding the molecular defects and unveiling therapeutic targets.
We are still facing several challenges and bottlenecks: 1) a number of patients are still awaiting molecular diagnosis; 2) relevant biomarkers are scarce; 3) functions of the involved proteins and underlying pathomechanisms are still poorly understood … We previously have and continue to tackle several transverse processes (e.g. contractile dysfunction, defective mechanosensing, fibrosis …) using our specific expertise (nuclear envelop, nucleoplasm, extracellular matrix…).
Current research axes:
- Definition of genetic and clinical spectrum and delineation of natural history of these NMDs,
- Development of new tools to validate genetic variants identified through NGS (next generation sequencing),
- Deciphering pathomechanisms that affect skeletal and/or cardiac muscle, with the overall goal of identifying and assessing therapeutic options for these disorders.
Our work is carried out on biological material derived from patients (DNA, RNA cultured cells, or muscle biopsies), and on animal models developed in the team (mouse, zebrafish).
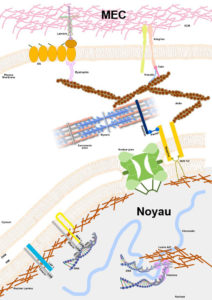
Noyau-MEC ©Astrid Brull
| Name | Position | ORCID |
|---|
- M Kammoun, V Veksler, J Piquereau, Gisèle Bonne, I Nelson, et al.. Loss of TIEG expression results in defective skeletal muscle structure and function with associated impairment of mitochondrial biogenesis.. Annual Meeting of the American-Society-for-Bone-and-Mineral-Research (ASBMR), Sep 2017, Denver (CO), United States. J. Bone Miner. Res., 32 (Suppl 1), pp.S7-S8. 1024, 2017. ⟨hal-03983941⟩
- Karim Wahbi, Caroline Chong-Nguyen, Vincent Algalarrondo, Henri Marc Becane, Pauline Arnaud, et al.. Association Between Mutation Size and Cardiac Involvement in Myotonic Dystrophy Type 1. International Myotonic Dystrophy Consortium Meeting IDMC-11, Sep 2017, San Francisco, United States. ⟨hal-04019185⟩
- Daniel Owens, Julien Messeant, G Herledan, Arnaud Ferry, Anne Bertrand, et al.. Nuclear envelope protein lamin A/C is a crucial mechanosensory component for skeletal muscle plasticity. International Congress of Neuromuscular Disorders, Sep 2017, Ottawa, Canada. 2017. ⟨hal-03968419⟩
- Gisèle Bonne. Maintenance of nucleoplasmic lamin A/C during myoblast differentiation induces nuclear fusion in LMNA-related congenital myopathy.. The Pleiotropic Nuclear Envelope, John McIntyre Conference Centre, University of Edinburgh, Aug 2017, Edinburgh (Ecosse), United Kingdom. ⟨hal-03972911⟩
- Felice Heller, Ivana Dabaj, Jean Mah, Jean Bergounioux, Aben Essid, et al.. Cardiac manifestations of congenital LMNA-related muscular dystrophy in children: three case reports and recommendations for care. Cardiology in the Young, 2017, 27 (6), pp.1076-1082. ⟨10.1017/S1047951116002079⟩. ⟨hal-03860662⟩
- Valérie Allamand. From Iowa City to Paris: From sarcolemma to extracellular matrix. Muscle Membrane Serendipity: Past, Present & Future, Jul 2017, Iowa City, United States. ⟨hal-04029249⟩
- Anne T. Bertrand. Development of gene therapy for LMNA-related congenital muscular dystrophy. Cure CMD scientific and family conference, Jul 2017, Washington DC, United States. ⟨hal-04004859⟩
- Gisèle Bonne. Insights in the pathophysiology of LMNA-related congenital muscular dystrophy. The Nuclear Lamina and nuclear organization. Cost School & The Batsheva de Rothschild Seminar., Jun 2017, Yearim, Israel. ⟨hal-03972905⟩
- Anne T Bertrand, Feriel Azibani, Bruno Cadot, Monica Zwerger, Colin Stewart, et al.. Maintenance of nucleoplasmic lamin A/C during myoblast differentiation induces nuclear fusion in LMNA-related congenital myopathy. 10th European meeting on Intermediate filaments, Jun 2017, Saint Malo, France. ⟨hal-03986879⟩
- R Rossi, C Scotton, M Lorenzo, A d'Amico, G Ricci, et al.. POPDC1 gene mutations screening in laminopathies: possible role as a modifier. 50th European-Society-of-Human-Genetics (ESHG) Conference, May 2017, Copenhagen, Denmark. Eur. J. Hum. Genet., 26 (S), pp.445-446. P10.51C, 2018. ⟨hal-03983938⟩
Our last work on the OJRD
A guide to writing systematic reviews of rare disease treatments to generate FAIRcompliant datasets: building a Treatabolome














