Repeat Expansions & Myotonic Dystrophy (REDs)
Description
The focus of the team’s research is centered on Myotonic Dystrophy, a common neuromuscular disease in adults, with a specific emphasis on DM type 1 (DM1), also known as Steinert’s disease. DM1 is characterised by a range of symptoms, including progressive muscle weakness and atrophy, myotonia, cardiac conduction defects, early cataracts, endocrine and gastrointestinal problems, and neurological impairment. This multisystemic disease displays clinical forms with differing ages of onset, such as the late, adult, juvenile, infantile and congenital forms of DM1. Currently, there is no treatment for this debilitating genetic disease, but various therapeutic approaches are under development.
DM1 is an autosomal dominant disease caused by an abnormal expansion of a (CTG)n trinucleotide repeat (n>40) located in the 3’ non-coding region of the DMPK gene. The unstable nature of the repetitive CTG expansions is detected in different tissues throughout the patient’s life, but also across successive generations, providing the molecular basis for the typical phenomenon of anticipation in DM1 families. The disease is mediated by a toxic RNA gain-of-function. The mutant DMPK transcripts containing pathological CUG repeat tracts (RNA-CUGexp) are retained in the nucleus of cells as riboprotein aggregates (called RNA foci), interfering with the function of specific RNA-binding proteins (RBPs). RNA foci specifically sequester RBPs of the MBNL family, which play a critical role in the maturation of RNAs. As a result, the loss of MBNL function leads to alternative splicing defects in certain pre-messenger RNAs. Some of these defects have been associated with various clinical symptoms, including those of CLCN1 to myotonia, INSR to insulin resistance, BIN1 to muscle weakness, DMD to alteration of muscle fibre architecture and SCN5A to cardiac defects. However, additional mechanisms contribute to the intricate pathophysiological processes of this complex condition, which affects a variety of cell types and numerous tissues.
The REDs team was created in 2019, by merging the former teams led by Geneviève Gourdon and Denis Furling. The team also includes the group of Guillaume Bassez, a neurologist who coordinates the national registry of myotonic dystrophy (DM-Scope) and that of Arnaud Ferry who is interested in muscle physiology and exercise. The objective of this new team is to synergize efforts to accelerate translational research for this neuromuscular disease and to bring out therapeutic alternatives for patients. To this end, the team implements integrated research, ranging from the fundamental mechanisms of the instability of CTG expansions, to the understanding of pathophysiological mechanisms using cellular and animal models, the development and evaluation of innovative therapeutic approaches and, finally, the establishment of pre-clinical and clinical trials for this DM1.
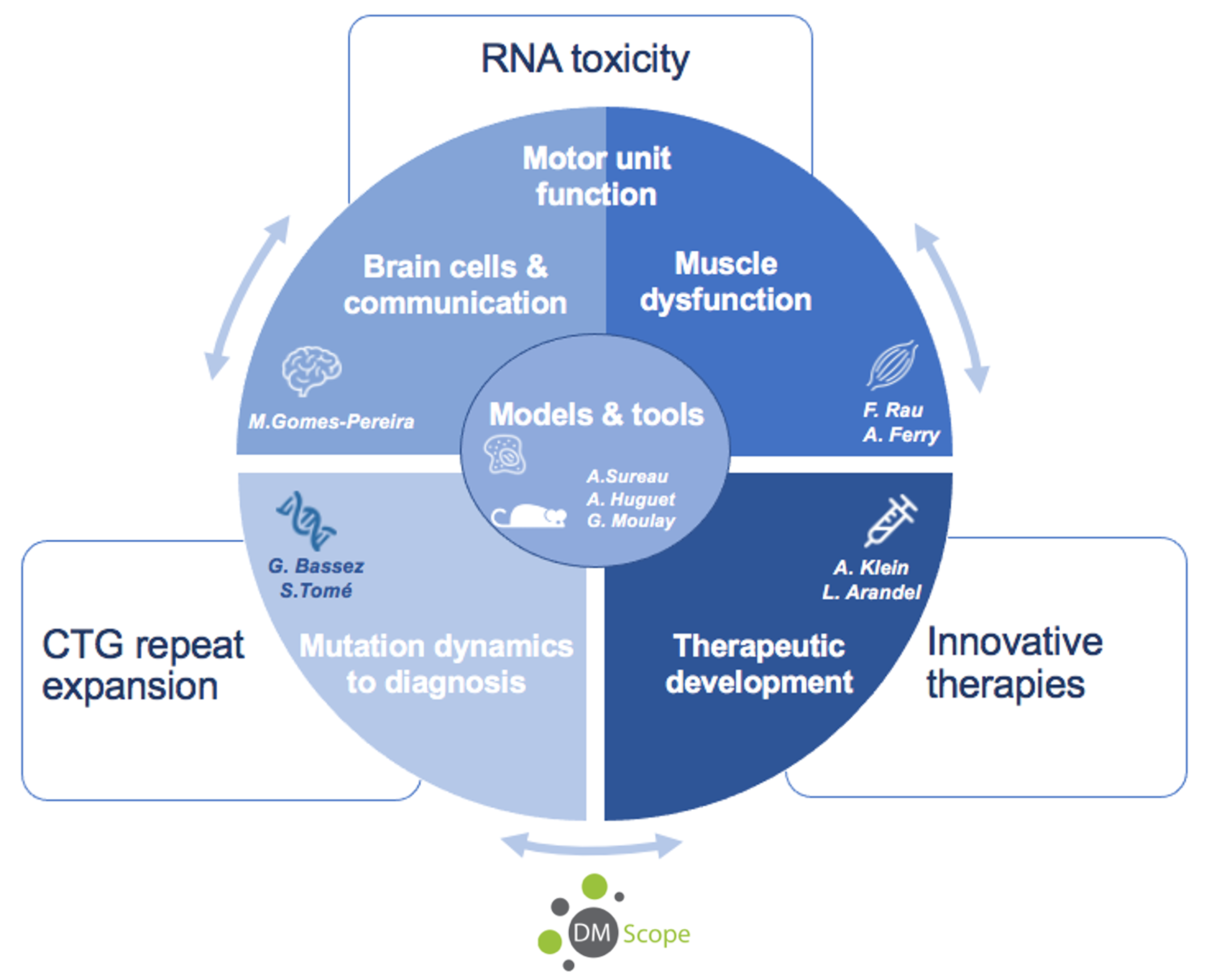
Research themes
Dynamics of CTG expansions and involvement for diagnosis (PI: G. Bassez & S. Tomé)
Using mouse and cellular models, we study the mechanisms of CTG repeat instability and the factors that modify their dynamics. In parallel, we investigate atypical DM1 families in connection with the DM-Scope patient registry and the neuromyology department. Crossing the data between the laboratory and the clinic will make possible to refine diagnostic and prognostic tools.
RNA toxicity and pathophysiological consequences
We investigate the impact of the repeat mutation and the expression toxic CUG RNA transcripts (CUGexpRNA) along the nervous system/muscle axis. In particular, we are very interested on:
- The molecular and functional consequences on different brain cells and on their communication (PI: M. Gomes-Pereira)
- The impact on motor neurons, motor unit and muscle function (PI: F. Rau & A. Ferry)
Development of new therapies (PIs: A. Klein & L. Arandel)
Our team has been developing various innovative therapeutic approaches for DM1, targeting different organs (muscle, brain, heart, etc.). The new strategies developed are first tested on cellular and mouse models of the disease, with the aim of direct transfer to the institute’s clinical services.
Models and tools (Leaders: A. Surreau, A. Huguet-Lachon & G. Moulay)
New cellular, mouse and molecular models and tools are developed by the team, in response to the progress of our research projects.
DM-Scope and clinical research (PI: G. Bassez)
Coordination of the French Observatory of Myotonic Dystrophies – DM-Scope –
- Phenotypic characterization and natural history of Myotonic Dystrophy
- Validation of measurement tools for clinical trials
- Clinical trial
Contacts :
| Name | Position | ORCID | Group |
|---|
- A. Ferry, S. Dugravot, Christides J.P., J. Auger, Bagneres A.G., et al.. How to use a general cue to find a specific resource : potential role of volatile concentration in prey location by ground dwelling predators of the cabbage root fly.. 23rd ISCE meeting, Jul 2007, JENA, Germany. pp.268. ⟨hal-00194802⟩
- Audrey Vignaud, F. Fougerousse, E. Mouisel, Christelle Bertrand, Béatrice Bonafos, et al.. Genetic ablation of acetylcholinesterase alters muscle function in mice. 9. International Meeting on Cholinesterases, May 2007, Suzhou, China. ⟨10.1016/j.cbi.2008.04.035⟩. ⟨hal-02758076⟩
- Christelle Bertrand, Béatrice Bonafos, Maud Tremblay, Arnaud Ferry, Arnaud Chatonnet. Effect of fluoxetine on neuromuscular function in acetylcholinesterase (AChE) knockout mice. 9. International Meeting on Cholinesterases, May 2007, Suzhou, China. ⟨10.1016/j.cbi.2008.04.002⟩. ⟨hal-02758520⟩
- Arnaud Chatonnet, Christelle Bertrand, Laurence Lepourry, Nathalie Barougier, Anne Ferry. Effect of fluoxetine on neuromuscular function in acetylcholinesterase (AChe) knockout mice. 9.International Meeting on Cholinesterases, May 2007, Suzhou, China. n.p. ⟨hal-02823585⟩
- Marc Bartoli, J. Poupiot, A. Vulin, F. Fougerousse, L. Arandel, et al.. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Therapy, 2007, 14 (9), pp.733-740. ⟨10.1038/sj.gt.3302928⟩. ⟨hal-01610042⟩
- J. Abraham, M. Aglietta, C. Aguirre, D. Allard, I. Allekotte, et al.. An upper limit to the photon fraction in cosmic rays above 10^19 eV from the Pierre Auger Observatory. Astroparticle Physics, 2007, 27, pp.155-168. ⟨10.1016/j.astropartphys.2006.10.004⟩. ⟨in2p3-00121304⟩
- A. Ferry, S. Dugravot, T. Delattre, Christides J.-P., J. Auger, et al.. Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground dwelling predators in the field.. Journal of Chemical Ecology, 2007, 33, pp.2064-2077. ⟨hal-00193705⟩
- Françoise Fougerousse, Marc Bartoli, Jérôme Poupiot, Ludovic Arandel, Muriel Durand, et al.. Phenotypic correction of alpha-sarcoglycan deficiency by intra-arterial injection of a muscle-specific serotype 1 rAAV vector. Molecular Therapy, 2007, 15 (1), pp.53-61. ⟨10.1038/sj.mt.6300022⟩. ⟨hal-01610043⟩
- A. Vignaud, F. Ramond, C. Hourdé, A. Keller, G. Butler-Browne, et al.. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice.. Pathobiology, 2007, 74 (5), pp.291-300. ⟨10.1159/000105812⟩. ⟨hal-00188014⟩
- J. Abraham, P. Abreu, M. Aglietta, C. Aguirre, D. Allard, et al.. Correlation of the highest-energy cosmic rays with nearby extragalactic objects. Science, 2007, 318, no 5852, pp.938-943. ⟨10.1126/science.1151124⟩. ⟨in2p3-00188862⟩












