Biotherapies for motor neuron disorders (ALS & SMA)
The main goal of our team is to develop new therapies for motor neuron disorders (MND). Our work is focused on spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).
The use of viral vectors derived from adeno associated virus (AAV) opened novel perspectives and applications for the treatment of MNDs. In 2007, M. Barkats demonstrated the high potential of self-complementary AAV serotype 9 (AAV9) to efficiently transduce the central nervous system (CNS) following a systemic injection (Barkats, Patent PCT/EP2008/063297, 2007 and publication Institut de Myologie). Remarkably, the first gene therapy based on this approach – Zolgensma® – has been recently approved by the Food and Drug Administration (FDA) for the treatment of infantile forms of SMA. This represents a major breakthrough in the field of gene therapy for rare diseases.
We are currently optimizing the AAV-mediated gene replacement approach for SMA. Our objective is to develop specific vectors targeting multiple organs affected in the disease (Besse et al., 2020). This will likely reduce the potential side-effects of the current therapy on the long term. We are also investigating epigenetic regulation in SMA and motor neuron degeneration. The study of epigenetic hallmarks will provide a comprehensive understanding of the disease and in particular of its different forms. Furthermore, this work will contribute to the identification of novel pathways implicated in the pathophysiology of SMA. The objective of these projects on the long term is to identify novel therapeutic targets, specific to each SMA patient and to design future personalized medicine approaches ( Smeriglio et al., 2020).
We are also taking advantage of the therapeutic potential of AAV vectors to find treatments for ALS. In 2017, we developed a therapeutic strategy for ALS caused by mutations in the superoxide dismutase 1 (SOD1) gene. Using an exon-skipping approach through AAV, we induced global decrease in the human mutant SOD1 in the SOD1G93A mouse model (Biferi et al., 2017). This work received the Prize4Life award “THE $1M AVI KREMER ALS TREATMENT PRIZE4LIFE”. We are currently furthering the pre-clinical development of this approach in collaboration with Généthon.
A big part of our research effort focuses on the development of a therapeutic strategy for ALS and fronto-temporal dementia (FTD) caused by mutations in C9ORF72 gene. This is the most common form of ALS (40% of familial forms and 7% of sporadic cases). The mutation results in a gain-of-function and a loss of C9ORF72 protein expression (Reviewed by Cappella et al., 2019). Our strategy aims to simultaneously target all the pathological mechanisms, using AAV vectors. We are also generating novel experimental models to better understand the disease.
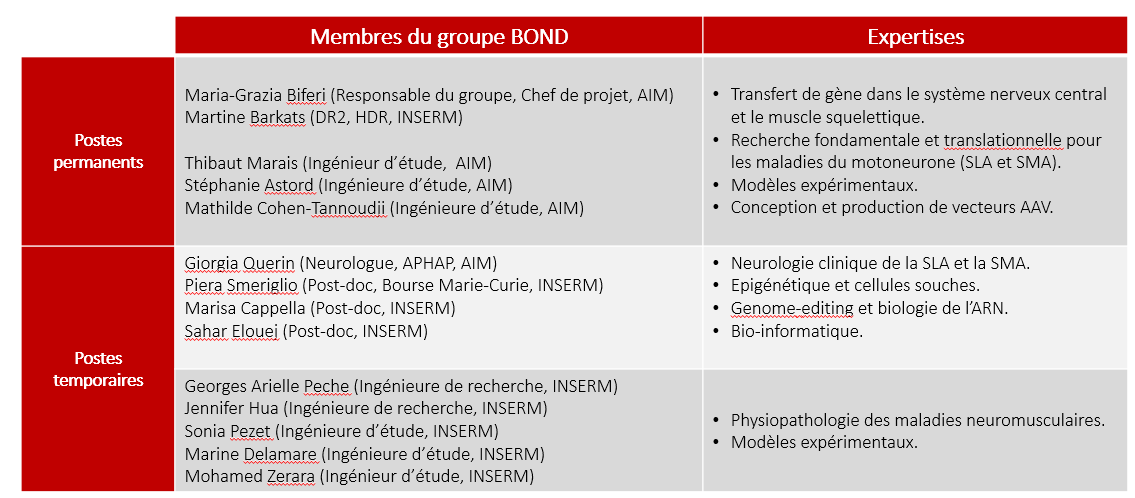
Contacts :
| Name | Position | ORCID | Group |
|---|
- Simon Newkirk, Suman Lee, Fiorella Grandi, Valeriya Gaysinskaya, James Rosser, et al.. Intact piRNA pathway prevents L1 mobilization in male meiosis. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114 (28), ⟨10.1073/pnas.1701069114⟩. ⟨hal-04199075⟩
- Aurore Besse, Mariane Roda, Stéphanie Astord, Thibaut Marais, Maria Grazia Biferi, et al.. SMN replacement restricted to the Central Nervous System does not rescue SMA mice.. Journée des familles, Jun 2017, Paris, France. ⟨hal-04003309⟩
- Aurore Besse. L’expression de SMN uniquement dans les neurones ne permet pas la survie des souris SMA. Journée des familles, Jun 2017, Paris, France. ⟨hal-04002581⟩
- Valerie Matagne, Yann Ehinger, Lydia Saidi, Ana Borges-Correia, Martine Barkats, et al.. A codon-optimized Mecp2 transgene corrects breathing deficits and improves survival in a mouse model of Rett syndrome. Neurobiology of Disease, 2017, 99, ⟨10.1016/j.nbd.2016.12.009⟩. ⟨hal-01426386⟩
- Anne-Sophie Gribling-Burrer, Michael Leichter, Laurence Wurth, Alexandra Huttin, Florence Schlotter, et al.. SECIS-binding protein 2 interacts with the SMN complex and the methylosome for selenoprotein mRNP assembly and translation. Nucleic Acids Research, 2017, 45 (9), pp.5399-5413. ⟨10.1093/nar/gkx031⟩. ⟨hal-01541890⟩
- Jieun Lee, Piera Smeriglio, Jason Dragoo, William Maloney, Nidhi Bhutani. CD24 enrichment protects while its loss increases susceptibility of juvenile chondrocytes towards inflammation. Arthritis Research & Therapy, 2016, 18 (1), pp.292. ⟨10.1186/s13075-016-1183-y⟩. ⟨hal-03818872⟩
- Piera Smeriglio, Sonia Alonso-Martin, Silvia Masciarelli, Luca Madaro, Ilaria Iosue, et al.. Phosphotyrosine phosphatase inhibitor bisperoxovanadium endows myogenic cells with enhanced muscle stem cell functions via epigenetic modulation of Sca-1 and Pw1 promoters. FASEB Journal, 2016, 30 (4), pp.1404-1415. ⟨10.1096/fj.15-275420⟩. ⟨hal-01515295⟩
- Nadine Dragin, Jacky Bismuth, Géraldine Cizeron-Clairac, Maria Grazia Biferi, Claire Berthault, et al.. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases.. Journal of Clinical Investigation, 2016, 126 (4), pp.1525-37. ⟨10.1172/JCI81894⟩. ⟨hal-01310502⟩
- Sarah E.B. Taylor, Jieun Lee, Piera Smeriglio, Adnan Razzaque, Robert Smith, et al.. Identification of Human Juvenile Chondrocyte-Specific Factors that Stimulate Stem Cell Growth. Tissue Engineering: Parts A, B, and C, 2016, 22 (7-8), pp.645-653. ⟨10.1089/ten.TEA.2015.0366⟩. ⟨hal-03818896⟩
- Sarah Eb Taylor, Ye Henry Li, Piera Smeriglio, Madhusikta Rath, Wing Wong, et al.. Stable 5-Hydroxymethylcytosine (5hmC) Acquisition Marks Gene Activation During Chondrogenic Differentiation. Journal of Bone and Mineral Research, 2016, 31 (3), pp.524-534. ⟨10.1002/jbmr.2711⟩. ⟨hal-03818918⟩