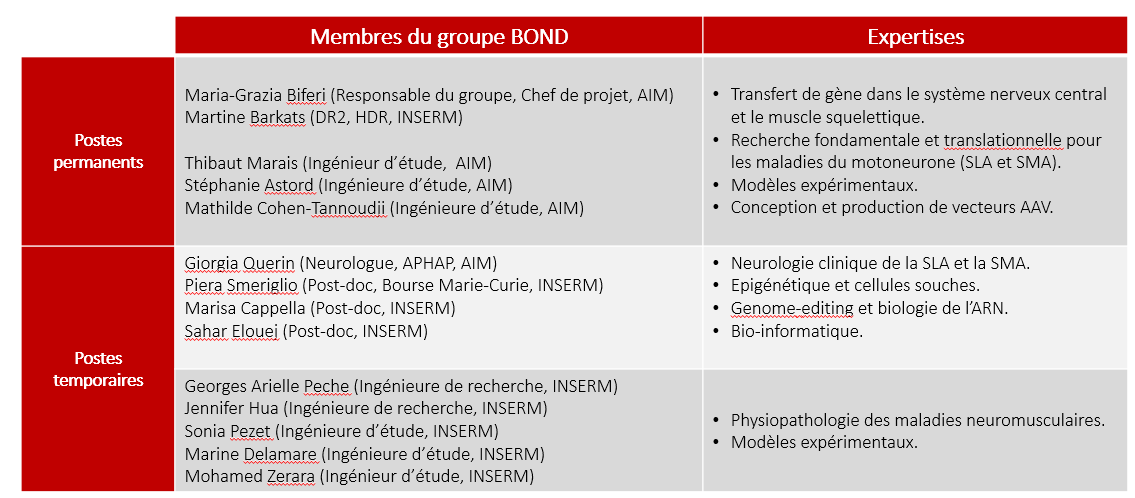
Biotherapies for motor neuron disorders (ALS & SMA)
The main goal of our team is to develop new therapies for motor neuron disorders (MND). Our work is focused on spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).
The use of viral vectors derived from adeno associated virus (AAV) opened novel perspectives and applications for the treatment of MNDs. In 2007, M. Barkats demonstrated the high potential of self-complementary AAV serotype 9 (AAV9) to efficiently transduce the central nervous system (CNS) following a systemic injection (Barkats, Patent PCT/EP2008/063297, 2007 and publication Institut de Myologie). Remarkably, the first gene therapy based on this approach – Zolgensma® – has been recently approved by the Food and Drug Administration (FDA) for the treatment of infantile forms of SMA. This represents a major breakthrough in the field of gene therapy for rare diseases.
We are currently optimizing the AAV-mediated gene replacement approach for SMA. Our objective is to develop specific vectors targeting multiple organs affected in the disease (Besse et al., 2020). This will likely reduce the potential side-effects of the current therapy on the long term. We are also investigating epigenetic regulation in SMA and motor neuron degeneration. The study of epigenetic hallmarks will provide a comprehensive understanding of the disease and in particular of its different forms. Furthermore, this work will contribute to the identification of novel pathways implicated in the pathophysiology of SMA. The objective of these projects on the long term is to identify novel therapeutic targets, specific to each SMA patient and to design future personalized medicine approaches ( Smeriglio et al., 2020).
We are also taking advantage of the therapeutic potential of AAV vectors to find treatments for ALS. In 2017, we developed a therapeutic strategy for ALS caused by mutations in the superoxide dismutase 1 (SOD1) gene. Using an exon-skipping approach through AAV, we induced global decrease in the human mutant SOD1 in the SOD1G93A mouse model (Biferi et al., 2017). This work received the Prize4Life award “THE $1M AVI KREMER ALS TREATMENT PRIZE4LIFE”. We are currently furthering the pre-clinical development of this approach in collaboration with Généthon.
A big part of our research effort focuses on the development of a therapeutic strategy for ALS and fronto-temporal dementia (FTD) caused by mutations in C9ORF72 gene. This is the most common form of ALS (40% of familial forms and 7% of sporadic cases). The mutation results in a gain-of-function and a loss of C9ORF72 protein expression (Reviewed by Cappella et al., 2019). Our strategy aims to simultaneously target all the pathological mechanisms, using AAV vectors. We are also generating novel experimental models to better understand the disease.
Contacts :
| Name | Position | ORCID | Group |
|---|
- Jieun Lee, Sarah Taylor, Piera Smeriglio, Janice Lai, William Maloney, et al.. Early induction of a prechondrogenic population allows efficient generation of stable chondrocytes from human induced pluripotent stem cells. FASEB Journal, 2015, 29 (8), pp.3399-3410. ⟨10.1096/fj.14-269720⟩. ⟨hal-03818924⟩
- Piera Smeriglio, Lakshmi Dhulipala, Janice Lai, Stuart Goodman, Jason Dragoo, et al.. Collagen VI Enhances Cartilage Tissue Generation by Stimulating Chondrocyte Proliferation. Tissue Engineering: Parts A, B, and C, 2015, 21 (3-4), pp.840-849. ⟨10.1089/ten.TEA.2014.0375⟩. ⟨hal-03818937⟩
- Piera Smeriglio, Janice Lai, Fan Yang, Nidhi Bhutani. 3D Hydrogel Scaffolds for Articular Chondrocyte Culture and Cartilage Generation. Journal of visualized experiments : JoVE, 2015, 104, ⟨10.3791/53085⟩. ⟨hal-03818902⟩
- Piera Smeriglio, Janice Lai, Lakshmi Dhulipala, Anthony Behn, Stuart Goodman, et al.. Comparative Potential of Juvenile and Adult Human Articular Chondrocytes for Cartilage Tissue Formation in Three-Dimensional Biomimetic Hydrogels. Tissue Engineering: Parts A, B, and C, 2015, 21 (1-2), pp.147-155. ⟨10.1089/ten.TEA.2014.0070⟩. ⟨hal-03818946⟩
- Giulia Maria Camerino, Marina Bouchè, Michela de Bellis, Maria Cannone, Antonella Liantonio, et al.. Protein kinase C theta (PKCθ) modulates the ClC-1 chloride channel activity and skeletal muscle phenotype: a biophysical and gene expression study in mouse models lacking the PKCθ. Pflügers Archiv European Journal of Physiology, 2014, 466 (12), pp.2215-2228. ⟨10.1007/s00424-014-1495-1⟩. ⟨hal-03818950⟩
- Sarah Taylor, Piera Smeriglio, Lakshmi Dhulipala, Madhusikta Rath, Nidhi Bhutani. A Global Increase in 5-Hydroxymethylcytosine Levels Marks Osteoarthritic Chondrocytes. Arthritis & rheumatology, 2014, 66 (1), pp.90-100. ⟨10.1002/art.38200⟩. ⟨hal-03818959⟩
- Vanessa Besson, Piera Smeriglio, Amélie Wegener, Frédéric Relaix, Brahim Nait Oumesmar, et al.. PW1 gene/paternally expressed gene 3 (PW1/Peg3) identifies multiple adult stem and progenitor cell populations. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108 (28), pp.11470-11475. ⟨10.1073/pnas.1103873108⟩. ⟨hal-03818968⟩
- Luca Madaro, Valeria Marrocco, Piera Fiore, Paola Aulino, Piera Smeriglio, et al.. PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase. Molecular Biology of the Cell, 2011, 22 (8), pp.1409-1419. ⟨10.1091/mbc.E10-10-0821⟩. ⟨hal-03818974⟩