Mario Gomes-Pereira, research scientist, Reds Team (Repeat Expansions & Myotonic Dystrophy) directed by Denis Furling and Geneviève Gourdon.
What is the general aim of this project?
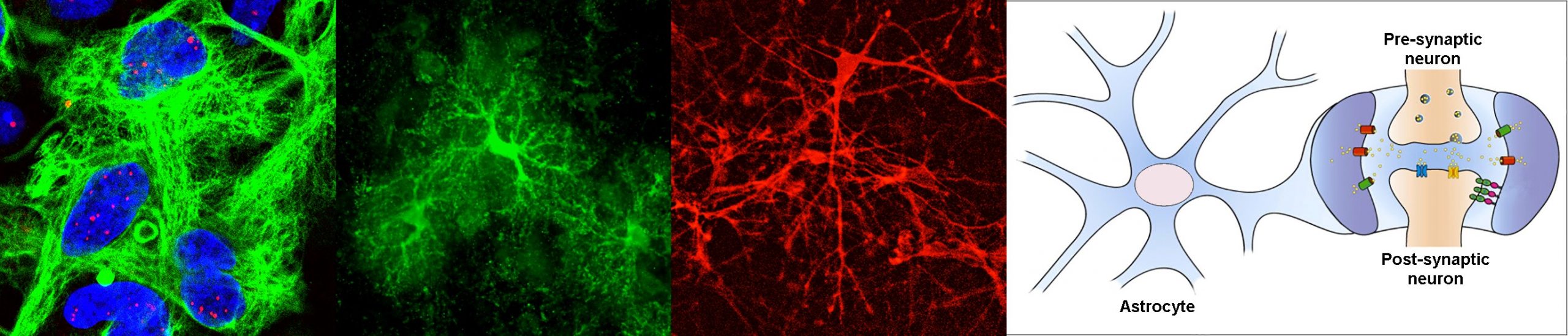
Myotonic dystrophy type 1, or DM1, is a neuromuscular disease that affects 1/8000 people worldwide, but this figure is likely underestimated. Although best known for its muscular manifestations, this multisystemic condition is characterised by prominent brain impairment. The DM1 mutation, a trinucleotide repeat expansion, was identified in 1992, and today we know a great deal about the molecular pathogenesis in muscle cells. In contrast, we still know little about the disease mechanisms in the central nervous system. The project stems from our hypothesis that DM1 has a dramatic impact on glial cells, with subsequent consequences on neuronal physiology and brain function. We will specifically investigate the mechanisms and consequences of astrocyte dysfunction in DM1, together with Cyril F. Bourgeois (ENS, Lyon), Cécile Martinat (I-Stem, Evry) and Nathalie Rouach (Collège de France, Paris).
Why have you decided to study the role of astrocytes in DM1?
When I joined the group of Geneviève Gourdon, we set a new axis on research to address the urgent need to understand brain disease mechanisms in DM1. I am today responsible for this line of research, which I have managed for the last 15 years. We first focused on the obvious target, the neurons, and found significant abnormalities in synaptic proteins [Brain (2013), 136: 957, DOI: 10.1093/brain/aws367]. However, we stumbled upon some intriguing and pronounced abnormalities in astrocytes too, which provided the first evidence of glial pathology [Cell Reports (2017) 19: 2718, DOI: 10.1016/j.celrep.2017.06.00; Frontiers in Cellular Neuroscience (2021), 15: 662035, DOI: 10.3389/fncel.2021.662035]. We were very intrigued by these surprising findings and decided to focus our attention on astrocytes. We demonstrated that the morphology, adhesion, orientation and migration of astrocytes is affected by DM1. These findings, recently published in Nature Communications, clearly shifted the neuro-centric view, towards a non-neuronal component in DM1 [Nature Communications (2022) 13: 3141. DOI: 10.1038/s41467-022-31594-9]. ASTROMYOD will further dissect the molecular mechanisms behind astrocyte pathology in DM1, and its functional impact on integrative brain function.
Which specific questions will you address and how will you get about to answer them?
Perturbations of RNA homeostasis are implicated in several human conditions, for which DM1 became the prototype. DM1 molecular pathogenesis involves the accumulation of toxic RNA transcripts in the cell nucleus, which alters primarily the activity of important RNA-binding proteins, such as the MBNL family of splicing regulators. MBNL proteins play a plethora of roles in RNA processing, including the regulation of alternative splicing, polyadenylation and intracellular trafficking of transcripts. Such features are extremely important in the highly ramified and compartmentalised cells of the brain, like the astrocytes. In this project we will investigate how the accumulation of toxic RNA disturbs astrocyte function through altered MBNL protein activity and defective RNA processing, and how these defects ultimately lead to brain dysfunction. We will combine subcellular transcriptomics, bioinformatics and high-resolution imaging to elucidate the mechanisms behind DM1 brain disease in unique transgenic mouse models of DM1, as well as in human pluripotent stem cells and organoids.
How was the research consortium put together?
The integrative investigation of the molecular and cellular mechanisms of brain disease requires a multidisciplinary approach. This project brings together four partners with complementary expertise in DM1 pathogenesis and animal models (M. Gomes-Pereira); bioinformatics of splicing and gene expression (C.F. Bourgeois); human pluripotent stem cell and organoid models (C. Martinat); astrocyte cell biology, electrophysiology and mouse behaviour (N. Rouach). This is not the first time we are collaborating. When I first decided to study astrocytes in DM1, I approached the other partners. We have been working together for the last 5 years. We previously obtained ANR funding to demonstrate for the first time astroglia defects in DM1, and we co-authored a couple of papers, with other manuscripts currently in preparation. It turned out to be a very successful and exciting collaboration. I am happy we have the financial means to take this story further.
What is the expected impact of your project?
The debilitating neuropsychological manifestations of DM1 translate into learning problems at school in young patients, as well as poor professional and socioeconomic integration later in life. Resolving brain pathogenesis is a priority for the development of effective therapies.
The involvement of glial cells in DM1 will shape the design of future therapies. To ameliorate the cognitive and behavioural deficits in DM1, the correction of neuronal defects alone will not be enough. It will be important to develop therapeutic tools that will also target astrocytes, and most probably other non-neuronal cells. Through the identification of the molecular pathways perturbed in DM1, and the development of new mouse and human disease models, we will establish the grounds and powerful tools to respond to the urgent therapeutic needs for this condition. Finally, our results will uncover important aspects of RNA biology in brain cells.