Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Our research interests focus on two groups of neuromuscular disorders (NMD): myopathies due to abnormalities of the myomatrix and of the nucleus. The long-term objective of our work is to propose relevant therapeutic options based on our knowledge of the genetic basis and of the underlying pathomechanisms at play in these rare diseases.
Research Project: Genetics and pathophysiology of neuromuscular disorders linked to the extracellular matrix and to the nucleus
Our team focuses on 2 groups of neuromuscular disorders: myopathies due to the defective myomatrix (collagen VI and other components of the extracellular matrix) and to defects of the myonucleus (Emery-Dreifuss muscular dystrophy and other striated muscle laminopathies due to mutations in the laminA/C gene or genes encoding components of nuclear membrane). These myopathies share some clinical features, notably prominent contractures, and constitute differential diagnosis[in1] .
These disorders are highly heterogeneous, clinically and genetically, and to date no treatment is available. Our previous work led us to identify the involvement of various genetic alterations and to develop tools (cellular and animal models) that are crucial for deciphering pathomechanisms, understanding the molecular defects and unveiling therapeutic targets.
We are still facing several challenges and bottlenecks: 1) a number of patients are still awaiting molecular diagnosis; 2) relevant biomarkers are scarce; 3) functions of the involved proteins and underlying pathomechanisms are still poorly understood … We previously have and continue to tackle several transverse processes (e.g. contractile dysfunction, defective mechanosensing, fibrosis …) using our specific expertise (nuclear envelop, nucleoplasm, extracellular matrix…).
Current research axes:
- Definition of genetic and clinical spectrum and delineation of natural history of these NMDs,
- Development of new tools to validate genetic variants identified through NGS (next generation sequencing),
- Deciphering pathomechanisms that affect skeletal and/or cardiac muscle, with the overall goal of identifying and assessing therapeutic options for these disorders.
Our work is carried out on biological material derived from patients (DNA, RNA cultured cells, or muscle biopsies), and on animal models developed in the team (mouse, zebrafish).
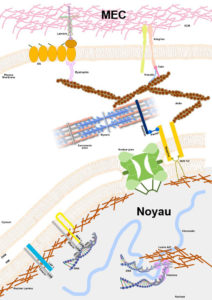
Noyau-MEC ©Astrid Brull
| Name | Position | ORCID |
|---|
- Allamand V, Laura Briñas, Pascale Richard, Tanya Stojkovic, Susana Quijano-Roy, et al.. ColVI-myopathies: where do we stand, where do we go?. Skeletal Muscle, 2011, 1 (1), pp.30. ⟨10.1186/2044-5040-1-30⟩. ⟨inserm-00630240⟩
- Mathieu Rederstorff, Perrine Castets, Sandrine Arbogast, Jeanne Lainé, Stéphane Vassilopoulos, et al.. Increased Muscle Stress-Sensitivity Induced by Selenoprotein N Inactivation in Mouse: A Mammalian Model for SEPN1-Related Myopathy. PLoS ONE, 2011, 6 (8), ⟨10.1371/journal.pone.0023094⟩. ⟨hal-01716017⟩
- Patricia Khattar, Felix Friedrich, Gisèle Bonne, Lucie Carrier, Thomas Eschenhagen, et al.. Distinction Between Two Populations of Islet-1-Positive Cells in Hearts of Different Murine Strains. Stem Cells and Development, 2011, 20 (6), pp.1043-1052. ⟨10.1089/scd.2010.0374⟩. ⟨hal-03824036⟩
- Perrine Castets, Anne T. Bertrand, Maud Beuvin, Arnaud Ferry, Fabien Le Grand, et al.. Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency.. Human Molecular Genetics, 2011, 20 (4), pp.694-704. ⟨10.1093/hmg/ddq515⟩. ⟨hal-00561402⟩
- B. Granger, L. Gueneau, V. Drouin-Garraud, Vincent Pedergnana, F. Gagnon, et al.. Modifier locus of the skeletal muscle involvement in Emery–Dreifuss muscular dystrophy. Human Genetics, 2011, 129 (2), pp.149-159. ⟨10.1007/s00439-010-0909-1⟩. ⟨hal-03691751⟩
- Rabah Ben Yaou, Claire L. Navarro, Susana Quijano-Roy, Anne T. Bertrand, Catherine Massart, et al.. Type B mandibuloacral dysplasia with congenital myopathy due to homozygous ZMPSTE24 missense mutation. European Journal of Human Genetics, 2011, ⟨10.1038/ejhg.2010.256⟩. ⟨hal-00611256⟩
- P. Castets, A.T. Bertrand, M. Beuvin, A. Ferry, F. Le Grand, et al.. O.3 Satellite cell loss is the pathomechanism leading to muscle atrophy in selenoprotein N deficiency. Neuromuscular Disorders, 2010, 20 (9-10), pp.598. ⟨10.1016/J.NMD.2010.07.012⟩. ⟨hal-02935581⟩
- Nadège Salvi, Aziz Guellich, Pierre Michelet, Alexandre Demoule, Morgan Le Guen, et al.. Upregulation of PPARβ/δ Is Associated with Structural and Functional Changes in the Type I Diabetes Rat Diaphragm. PLoS ONE, 2010, 5 (7), pp.e11494. ⟨10.1371/journal.pone.0011494⟩. ⟨inserm-02426556⟩
- Alain Lescure, Mathieu Rederstorff, Alain Krol, Pascale Guicheney, Valérie Allamand. Selenoprotein function and muscle disease. Biochimica et Biophysica Acta (BBA) - General Subjects, 2009, 1790 (11), pp.1569 - 1574. ⟨10.1016/j.bbagen.2009.03.002⟩. ⟨hal-01716049⟩
- Alain Lescure, Mathieu Rederstorff, Alain Krol, Pascale Guicheney, Valérie Allamand. Selenoprotein function and muscle disease. Biochimica et Biophysica Acta (BBA) - General Subjects, 2009, 1790 (11), pp.1569-1574. ⟨10.1016/j.bbagen.2009.03.002⟩. ⟨hal-03844559⟩
Our last work on the OJRD
A guide to writing systematic reviews of rare disease treatments to generate FAIRcompliant datasets: building a Treatabolome














